Does a Liquid Have More Energy Than a Solid
Heat is probably the easiest energy you can use to change your physical state. And obviously there is no solid form of hydrogen and methane.
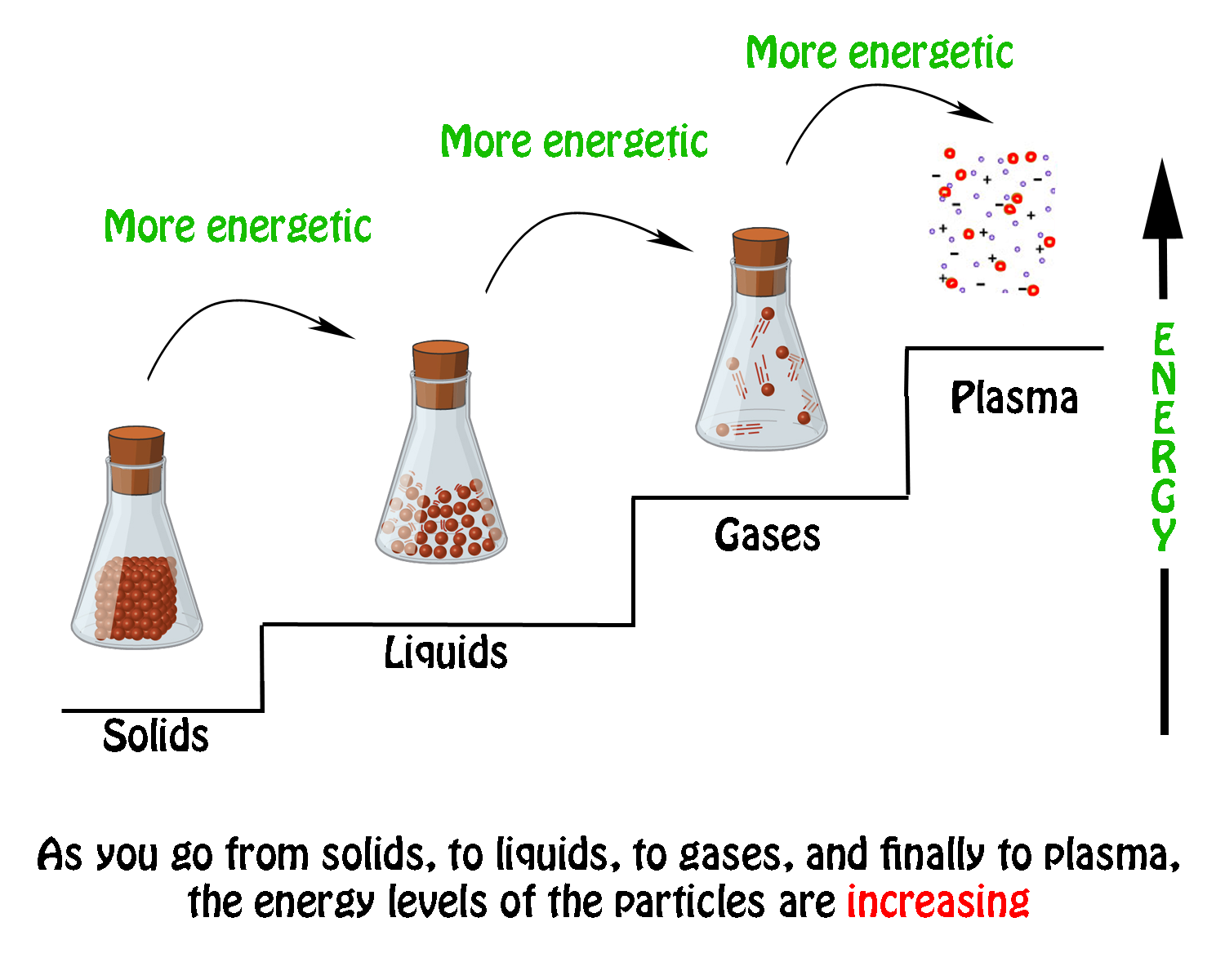
For The Three States Of Matter Solid Liquid And Gas There Are Six Possible Changes Of State Which Changes Of State Are Exothermic And Which Are Endothermic Socratic
Solid to a Liquid and Back to a Solid Imagine that you are a solid.
. Because they are moving faster the particles in the liquid occupy more space and the liquid is less dense than the corresponding solid. In fact solids often have 6 degrees of freedom because interaction with the surrounding atoms means that positional degrees of freedom also have a quadratic energy dependence and so count towards the total degrees of freedom. The same volume of substance X as solid and liquid may have different numbers of molecules so the liquid form could have equal or less.
The molecules move around very little and have a low amount of energy. The boiled water now in a gaseous state is really hot and can be considered full of potential energy. Therefore molecules of the same substance as the solid that are in the gas state would have a higher level of thermal energy than both the liquid state and the solid state of the same molecules.
You need some energy. You dream of becoming liquid water. So if we keep the number of moles molecules the same in the solid and liquid in question then theoretically yes.
Those same elements hydrogen and methane if you get them in a liquid state through use of higher pressures if you ignite that liquid it will release even more energy than their gas forms. Youre a cube of ice sitting on a counter. Assuming you mean a solid and liquid of different has to be different materials.
The atoms in a liquid have more energy than the atoms in a solid. If therefore the equipartition theorem was the only factor then the solid would have more internal energy than the gas. A mass of substance X in solid form has less energy than the same mass of substance X in liquid form because in liquids the molecules are moving more and thus have more kinetic energy.
When you boil water the H2O molecules go from a liquid state to a gas stateTo do this you need to heat the water up. If you add heat energy to a liquid the particles will move faster around each other as their kinetic energy increases. The solid having more molecules has.
But we cant ignore the chemical properties of the substances interactions between molecules if were to. Liquids have more kinetic energy than solids. Well keep it simple and say energy is the same thing as heat not entirely true but for the sake of this explanation it will suffice.
Molecules in a liquid have more energy than molecules in a solid. There are some times this rule doesnt quite work. On average the solids molecules have the same kinetic energy as the liquid.
Going from liquid to a solid does the same thing. The closest hydrogen gets to a solid is when subjected to pressures like those found close to the core of Jupiter. This frees up energy while the loss of entropydisorder in the liquid allows the higher degree of order that is needed to overlap orbitals via the EM force.
The liquid has higher entropy and when it freezes this disorder is lowered. There is more solid than liquid. The particles in a liquid have more kinetic energy than the particles in the corresponding solid.
Temperature is a measure proportional to the average kinetic energy of each particle. If you add energy by heating it up the molecules will move around faster and slide against each other and it will be a liquid. As a result the particles in a liquid move faster in terms of vibration rotation and translation.
And if you heat it up even more the molecules will speed up so much that they wont be stuck together at all. If you add heat energy to a solid the particles will vibrate with larger and larger amplitudes wobbles and eventually more and more of these particles will be able to break their solid bonds to form a liquid melting.

Particle Theory Changes Of State
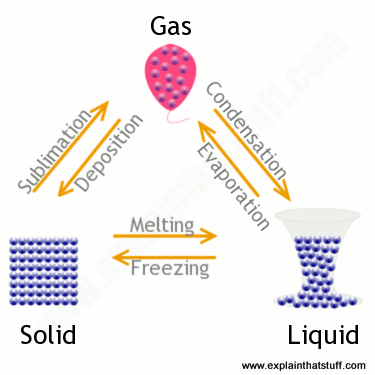
States Of Matter A Simple Introduction To Solids Liquids Gases

Comments
Post a Comment